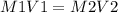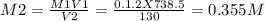Chemistry, 23.06.2019 02:00 FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with...
Questions

Physics, 14.04.2021 17:40




Mathematics, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40



Mathematics, 14.04.2021 17:40


Arts, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40

Computers and Technology, 14.04.2021 17:40



Mathematics, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40