
Chemistry, 22.06.2019 19:30 youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of...
Questions



Physics, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00

Biology, 10.07.2019 20:00


Social Studies, 10.07.2019 20:00

Social Studies, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00

Advanced Placement (AP), 10.07.2019 20:00

Mathematics, 10.07.2019 20:00


Mathematics, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00




Mathematics, 10.07.2019 20:00


Mathematics, 10.07.2019 20:00

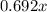 grams.
grams.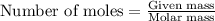 ....(1)
....(1)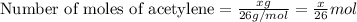
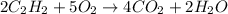
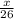 moles of acetylene will produce =
moles of acetylene will produce = 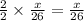 moles of water.
moles of water.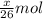
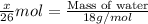
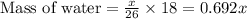 grams
grams 

