Chemistry, 22.06.2019 06:30 khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution...
Questions



Geography, 16.01.2020 13:31

Mathematics, 16.01.2020 13:31


Mathematics, 16.01.2020 13:31

Mathematics, 16.01.2020 13:31

Mathematics, 16.01.2020 13:31


English, 16.01.2020 13:31



Mathematics, 16.01.2020 13:31



Mathematics, 16.01.2020 13:31


Mathematics, 16.01.2020 13:31

History, 16.01.2020 13:31

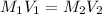
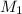 = molarity of KOH solution
= molarity of KOH solution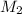 = molarity of new solution
= molarity of new solution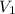 = volume of KOH solution
= volume of KOH solution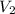 = volume of new solution
= volume of new solution