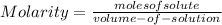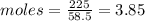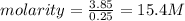Chemistry, 22.06.2019 04:00 lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of sol...
Questions

Mathematics, 12.11.2020 19:40

English, 12.11.2020 19:40

Chemistry, 12.11.2020 19:40

Arts, 12.11.2020 19:40

Mathematics, 12.11.2020 19:40


Biology, 12.11.2020 19:40



Mathematics, 12.11.2020 19:40

Mathematics, 12.11.2020 19:40


Mathematics, 12.11.2020 19:40

Mathematics, 12.11.2020 19:40


Social Studies, 12.11.2020 19:40


Mathematics, 12.11.2020 19:40

Mathematics, 12.11.2020 19:40