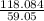Chemistry, 21.06.2019 15:30 hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2


Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen...
Questions

Mathematics, 24.12.2019 02:31



Biology, 24.12.2019 02:31



Biology, 24.12.2019 02:31


English, 24.12.2019 02:31

Mathematics, 24.12.2019 02:31

Social Studies, 24.12.2019 02:31


Mathematics, 24.12.2019 02:31

Mathematics, 24.12.2019 02:31





Spanish, 24.12.2019 02:31

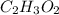 and molecular formula is
and molecular formula is 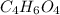 .
. 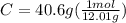
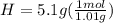
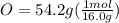
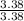 = 1
= 1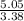 = 1.5
= 1.5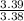 = 1
= 1