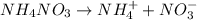Commercial cold packs often contain solid nh4no3 and a pouch of water. the temperature of the pack drops as the nh4no3 dissolves in water. therefore, for the dissolving of nh4no3 in water, a. δhsoln is positive and δssoln may be negative or positive. b. δhsoln is positive and δssoln is positive. c. δhsoln is negative and δssoln is positive. d. δhsoln is negative and δssoln may be negative or positive.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Commercial cold packs often contain solid nh4no3 and a pouch of water. the temperature of the pack d...
Questions

Mathematics, 30.08.2021 04:50




History, 30.08.2021 04:50

Biology, 30.08.2021 04:50

Mathematics, 30.08.2021 04:50



English, 30.08.2021 04:50

Mathematics, 30.08.2021 05:00

English, 30.08.2021 05:00

Mathematics, 30.08.2021 05:00



Mathematics, 30.08.2021 05:00


Computers and Technology, 30.08.2021 05:00

History, 30.08.2021 05:00

Advanced Placement (AP), 30.08.2021 05:00

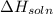 is positive and
is positive and 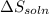 is positive.
is positive.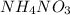 in water. Thus as the energy has been absorbed in the reaction, the reaction is endothermic and the change in enthalpy i.e.
in water. Thus as the energy has been absorbed in the reaction, the reaction is endothermic and the change in enthalpy i.e.