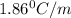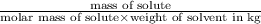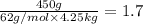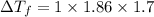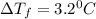Ethylene glycol (molar mass = 62 g/mol)is used as an antifreeze in cars. if 450 g of ethylene glycol is added to 4.25 kg of water, what is the molality? calculate how much the freezing point of water will be lowered. the freezing point depression constant for water is kf = -1.86°c/m. show your work.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
Ethylene glycol (molar mass = 62 g/mol)is used as an antifreeze in cars. if 450 g of ethylene glycol...
Questions

Mathematics, 10.12.2021 05:40

History, 10.12.2021 05:40


Mathematics, 10.12.2021 05:40

Physics, 10.12.2021 05:40


Mathematics, 10.12.2021 05:40


Physics, 10.12.2021 05:40



Spanish, 10.12.2021 05:40


Mathematics, 10.12.2021 05:40



Physics, 10.12.2021 05:40

Mathematics, 10.12.2021 05:40

Physics, 10.12.2021 05:40

Biology, 10.12.2021 05:40

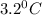
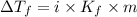
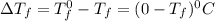 = Depression in freezing point
= Depression in freezing point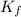 = freezing point constant =
= freezing point constant =