
Chemistry, 29.10.2019 13:31 mahaleyrenee1195
Trimix 10/50 is a gas mixture that contians 10% oxygen and 50% helium, and the rest is nitrogen. if a tank of trimix 10/50 has a total pressure of 2.07 x 104 kpa, then what is the partial pressure of helium?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1


Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
Trimix 10/50 is a gas mixture that contians 10% oxygen and 50% helium, and the rest is nitrogen. if...
Questions

Mathematics, 05.03.2021 22:10




Mathematics, 05.03.2021 22:10

Biology, 05.03.2021 22:10

Mathematics, 05.03.2021 22:10

English, 05.03.2021 22:10


Chemistry, 05.03.2021 22:10

Mathematics, 05.03.2021 22:10

Physics, 05.03.2021 22:10



Biology, 05.03.2021 22:10

Arts, 05.03.2021 22:10




History, 05.03.2021 22:10

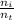
 = 0.313 mol
= 0.313 mol = 6.246 mol
= 6.246 mol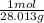 = 1.428 mol
= 1.428 mol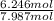 = 0.78
= 0.78

