Chemistry, 19.12.2019 11:31 erinloth123
Aph scale with ph values of some aqueous solutions. look at the figure showing the ph values of some familiar aqueous solutions. what is the difference between h+ concentration in an acidic solution such as lemon juice (ph 2) and a basic solution such as household bleach (ph 13)? a ph scale with ph values of some aqueous solutions. look at the figure showing the ph values of some familiar aqueous solutions. what is the difference between h+ concentration in an acidic solution such as lemon juice (ph 2) and a basic solution such as household bleach (ph 13)? the h+ concentration of lemon juice is higher than the h+ concentration of household bleach by a factor of 11. the h+ concentration of lemon juice is higher than the h+ concentration of household bleach by a factor of 1011 (100 billion). the h+ concentration of household bleach is higher than the h+ concentration of lemon juice by a factor of 1011 (100 billion).

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 07:30
Can you guys answer these questions i need it before 1: 00pm
Answers: 3

Chemistry, 23.06.2019 12:00
Explaining why atoms bondcomplete the sentence.atoms form chemical bonds to satisfy the rule and to become .
Answers: 1

Chemistry, 23.06.2019 15:30
Among these processes, which is the slowest chemical reaction? a. digesting food b. boiling an egg c. tarnishing of silver d. melting of a glacier
Answers: 2
You know the right answer?
Aph scale with ph values of some aqueous solutions. look at the figure showing the ph values of some...
Questions

Medicine, 21.05.2021 20:00






English, 21.05.2021 20:00

History, 21.05.2021 20:00

English, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00





French, 21.05.2021 20:00

History, 21.05.2021 20:00



Mathematics, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00

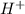 concentration of lemon juice is higher than the
concentration of lemon juice is higher than the 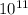 (100 billion)
(100 billion)![pH=-log[H^+]](/tpl/images/0425/9876/15713.png)
![2=-log[H^+]](/tpl/images/0425/9876/a01ca.png)
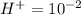
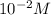 of
of ![13=-log[H^+]](/tpl/images/0425/9876/f4c07.png)
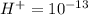
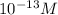 of
of