
Chemistry, 17.10.2019 01:00 HighSchool97654
How many moles of water are produced when 3.0 moles of hydrogen gas react with 1.8 moles of oxygen gas?
a. 3.0 moles of water
b. 3.6 moles of water
c. 5.3 moles of water
d. 7.0 moles of water

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
How many moles of water are produced when 3.0 moles of hydrogen gas react with 1.8 moles of oxygen g...
Questions









Biology, 28.02.2020 23:28






History, 28.02.2020 23:28





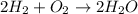
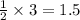 moles of oxygen.
moles of oxygen.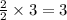 moles of water.
moles of water.

