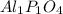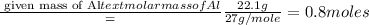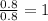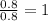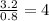Chemistry, 01.10.2019 20:00 cami30031cami3003
What is the empirical formula for 22.1% al, 25.4% p, 52.5% o

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

You know the right answer?
What is the empirical formula for 22.1% al, 25.4% p, 52.5% o...
Questions

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Chemistry, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

English, 14.07.2020 01:01


Geography, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Chemistry, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01



English, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Computers and Technology, 14.07.2020 01:01


Geography, 14.07.2020 01:01