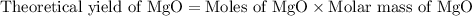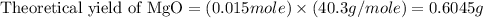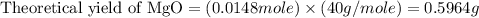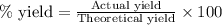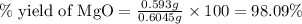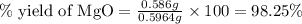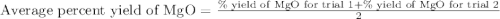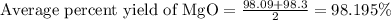Chemistry, 10.11.2019 17:31 shortty1111
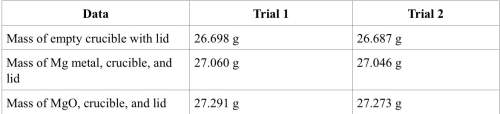
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial 2:
2. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial.
trial 1:
trial 2:
3. determine the percent yield of mgo for your experiment for each trial.
trial 1:
trial 2:
4. determine the average percent yield of mgo for the two trials.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1
You know the right answer?
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial...
trial 1:
trial...
Questions


History, 05.07.2019 15:00



Computers and Technology, 05.07.2019 15:00



Computers and Technology, 05.07.2019 15:00



Mathematics, 05.07.2019 15:00


English, 05.07.2019 15:00



English, 05.07.2019 15:00




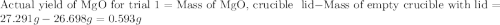
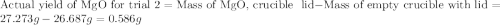
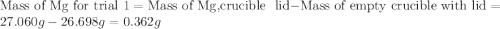
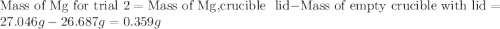
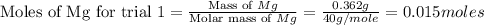
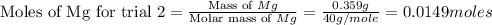
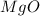 for trial 1 and trial 2.
for trial 1 and trial 2.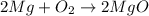
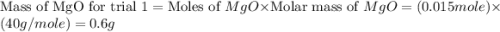
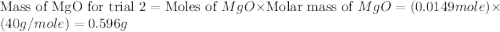
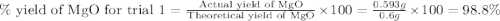
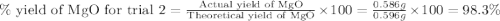

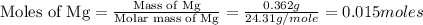
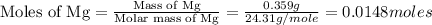
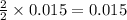 moles of MgO.
moles of MgO.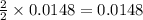 moles of MgO.
moles of MgO.