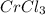The chemical equation for a reaction between k2cr2o7 and hcl is shown. k2cr2o7 + 14hcl → 2crcl3 + 2kcl + 3cl2 + 7h2o
which of the following identifies the reactant that acts as an oxidizing agent in the reaction and explains the answer? 20 !
a. k2cr2o7, because the oxidation number of k changes from +6 to +3.
b. k2cr2o7, because the oxidation number of cr changes from +6 to +3.
c. hcl, because the oxidation number of h changes from −1 to 0.
d. hcl, because the oxidation number of cl changes from −1 to 0.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1


Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 15:00
Solve this problem using the appropriate law. (remember that ) what is the pressure of 1.9 mols of nitrogen gas in a 9.45 l tank and at a temperature of 228 k?
Answers: 1
You know the right answer?
The chemical equation for a reaction between k2cr2o7 and hcl is shown. k2cr2o7 + 14hcl → 2crcl3 + 2k...
Questions


Mathematics, 28.05.2021 17:10


Mathematics, 28.05.2021 17:10

Mathematics, 28.05.2021 17:10

Mathematics, 28.05.2021 17:10



Mathematics, 28.05.2021 17:10



Mathematics, 28.05.2021 17:10



History, 28.05.2021 17:10



English, 28.05.2021 17:10


English, 28.05.2021 17:10

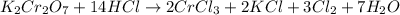
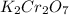 acts like and oxidizing agent because it is itself getting reduced to
acts like and oxidizing agent because it is itself getting reduced to