
Chemistry, 12.10.2019 00:50 sierravick123owr441
N2 (g) + 3 h2 (g) ? 2nh3 (g) ? h = -92 kj/mol a. is this reaction exothermic or endothermic? b. what direction will the equilibrium shift if nitrogen gas is removed? c. what direction will the equilibrium shift if the temperature is lowered? d. what direction will the equilibrium shift if ammonia (nh3) is added? e. what principle you answer b-d?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
N2 (g) + 3 h2 (g) ? 2nh3 (g) ? h = -92 kj/mol a. is this reaction exothermic or endothermic? b. wh...
Questions


English, 22.06.2019 22:00


History, 22.06.2019 22:00


Mathematics, 22.06.2019 22:00

Mathematics, 22.06.2019 22:00



Mathematics, 22.06.2019 22:00

Mathematics, 22.06.2019 22:00




Mathematics, 22.06.2019 22:00

Mathematics, 22.06.2019 22:00



History, 22.06.2019 22:00

English, 22.06.2019 22:00

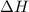 of the reaction is negative and it indicates an exothermic reaction.
of the reaction is negative and it indicates an exothermic reaction.

