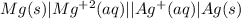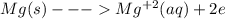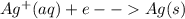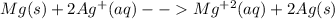An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) || aq^+(aq) | aq(s)
write a balanced redox equation for the cell using the oxidation and reduction half-reactions. (be sure to equalize charge by multiplying by the correct numbers before adding and simplifying)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) ||...
mg(s) | mg^2+ (aq) ||...
Questions


History, 08.07.2019 23:30


Mathematics, 08.07.2019 23:30



Mathematics, 08.07.2019 23:30

Mathematics, 08.07.2019 23:30

Mathematics, 08.07.2019 23:30

History, 08.07.2019 23:30

Mathematics, 08.07.2019 23:30

Mathematics, 08.07.2019 23:30

Biology, 08.07.2019 23:30



Mathematics, 08.07.2019 23:30

Mathematics, 08.07.2019 23:30

Mathematics, 08.07.2019 23:30