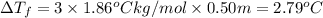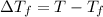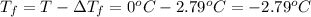Chemistry, 21.10.2019 17:30 KindaSmartPersonn
What is the expected freezing point of a 0.50 m solution of li2so4 in water? kf for water is 1.86 ∘c/m.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1



Chemistry, 24.06.2019 05:00
Ajeweler is making sapphire rings using 2 sapphires on every 1 ring. if the jeweler has 12 sapphires and 10 rings, what is the theoretical yield?
Answers: 1
You know the right answer?
What is the expected freezing point of a 0.50 m solution of li2so4 in water? kf for water is 1.86 ∘...
Questions


Mathematics, 28.01.2020 19:05




Mathematics, 28.01.2020 19:05


Geography, 28.01.2020 19:05

World Languages, 28.01.2020 19:05

Social Studies, 28.01.2020 19:05


Mathematics, 28.01.2020 19:05

Health, 28.01.2020 19:05

Mathematics, 28.01.2020 19:05



Mathematics, 28.01.2020 19:05

Mathematics, 28.01.2020 19:05


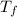
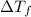
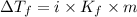
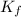 = The freezing point depression constant
= The freezing point depression constant