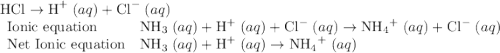Chemistry, 18.12.2019 10:31 lezapancakes13
Which of the chemical equations below are acid-base (proton transfer) reactions? select all that apply. mg(oh)2 (s) + h2so4 (aq) → mgso4 (s) + 2 h2o (l) fe(no3)3 (aq) + 3 koh (aq) → fe(oh)3 (s) + 3 kno3 (aq) zn (s) + cu(no3)2 (aq) → zn(no3)2 (aq) + cu (s) mg (s) + cu(no3)2 (aq) --> mg(no3)3 (aq) + cu (s) hcl (aq) + koh (aq) → kcl (aq) + h2o (l) nh3 (aq) + hcl (aq) → nh4cl (aq)?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Which of the chemical equations below are acid-base (proton transfer) reactions? select all that ap...
Questions

Mathematics, 30.07.2019 20:30


Mathematics, 30.07.2019 20:30




Chemistry, 30.07.2019 20:30

History, 30.07.2019 20:30

English, 30.07.2019 20:30







English, 30.07.2019 20:30



Computers and Technology, 30.07.2019 20:30