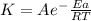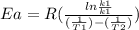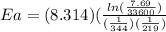Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction. a reaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction. 12.5 kj/mol 11.5 kj/mol 23.8 kj/mol 58.2 kj/mol 42.0 kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1


Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

You know the right answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions




Mathematics, 21.04.2020 06:42

Biology, 21.04.2020 06:42


Mathematics, 21.04.2020 06:43


English, 21.04.2020 06:43



Health, 21.04.2020 06:43


History, 21.04.2020 06:43




Biology, 21.04.2020 06:44


Mathematics, 21.04.2020 06:44