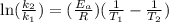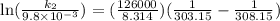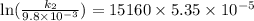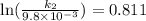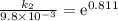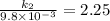Chemistry, 22.01.2020 23:31 19thomasar
The aquation of tris(1,10-phenanthroline)iron(ii) in acid solution takes place according to the equation: fe(phen)32+ + 3 h3o+ + 3 h2o → fe(h2o)62+ + 3 phenh+. if the activation energy, ea, is 126 kj/mol and the rate constant at 30°c is 9.8 × 10-3 min-1, what is the rate constant at 35°c? the aquation of tris(1,10-phenanthroline)iron(ii) in acid solution takes place according to the equation: fe(phen)32+ + 3 h3o+ + 3 h2o → fe(h2o)62+ + 3 phenh+. if the activation energy, ea, is 126 kj/mol and the rate constant at 30°c is 9.8 × 10-3 min-1, what is the rate constant at 35°c? 4.4 × 10-3 min-1 4.5 × 101 min-1 2.3 × 102 min-1 2.2 × 10-2 min-1

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

You know the right answer?
The aquation of tris(1,10-phenanthroline)iron(ii) in acid solution takes place according to the equa...
Questions

Arts, 03.12.2020 22:00




Computers and Technology, 03.12.2020 22:00



Biology, 03.12.2020 22:00



Mathematics, 03.12.2020 22:00



Mathematics, 03.12.2020 22:00




Mathematics, 03.12.2020 22:00

Mathematics, 03.12.2020 22:00

Chemistry, 03.12.2020 22:00