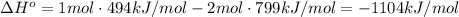Chemistry, 31.10.2019 11:31 24hudsonmoss
Given: c + o2 → co2 bond bond energy (kj/mol) c=o 799 o=o 494 calculate the enthalpy change for the chemical reaction. the change in enthalpy for the given reaction is kilojoules.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Given: c + o2 → co2 bond bond energy (kj/mol) c=o 799 o=o 494 calculate the enthalpy change for the...
Questions

History, 06.05.2020 06:06

Mathematics, 06.05.2020 06:06

Social Studies, 06.05.2020 06:06

English, 06.05.2020 06:06



English, 06.05.2020 06:06

Mathematics, 06.05.2020 06:06




Mathematics, 06.05.2020 06:06


English, 06.05.2020 06:06

Mathematics, 06.05.2020 06:06


Mathematics, 06.05.2020 06:06

Biology, 06.05.2020 06:06


Mathematics, 06.05.2020 06:07