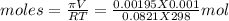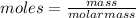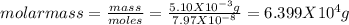Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1

Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1
You know the right answer?
Asolution was made by dissolving 5.10 mg of hemoglobin in water to give a final volume of 1.00 ml. t...
Questions

Mathematics, 19.02.2021 01:00

History, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00







Biology, 19.02.2021 01:00

Social Studies, 19.02.2021 01:00


Computers and Technology, 19.02.2021 01:00



History, 19.02.2021 01:00


Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00