
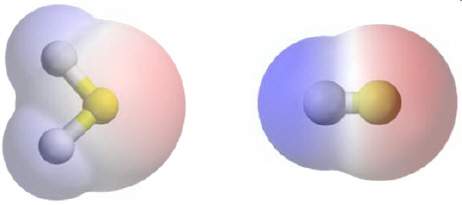
In the molecules below, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue. the attractive force between these two molecules has most likely been produced by covalent bonds. dipole-dipole interactions. dipole-induced dipole interactions. london dispersion forces.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
In the molecules below, areas that have a partial negative charge are pink and areas that have a par...
Questions




History, 31.01.2020 21:54




Mathematics, 31.01.2020 21:54



History, 31.01.2020 21:54

Mathematics, 31.01.2020 21:55


Biology, 31.01.2020 21:55

Mathematics, 31.01.2020 21:55




Physics, 31.01.2020 21:55




