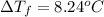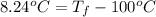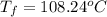Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1


Chemistry, 23.06.2019 11:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 300j of energy to the room?unit:
Answers: 1
You know the right answer?
Calculate the boiling point of a solution of 500.0 g of ethylene glycol (c2h6o2) dissolved in 500.0...
Questions

Mathematics, 04.11.2020 21:40

Mathematics, 04.11.2020 21:40


Mathematics, 04.11.2020 21:40



English, 04.11.2020 21:40

English, 04.11.2020 21:40



Mathematics, 04.11.2020 21:40




History, 04.11.2020 21:40

Mathematics, 04.11.2020 21:40

Spanish, 04.11.2020 21:40

SAT, 04.11.2020 21:40

Mathematics, 04.11.2020 21:40