

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Calculate the mass (g) of agcl formed when 174 g of silver sulfide reacts with excess hydrochloric a...
Questions

Mathematics, 17.05.2021 19:40

Mathematics, 17.05.2021 19:40


English, 17.05.2021 19:40

Computers and Technology, 17.05.2021 19:40

Social Studies, 17.05.2021 19:40



English, 17.05.2021 19:40

Mathematics, 17.05.2021 19:40

Mathematics, 17.05.2021 19:40

History, 17.05.2021 19:40

Mathematics, 17.05.2021 19:40

English, 17.05.2021 19:40



Mathematics, 17.05.2021 19:40

Mathematics, 17.05.2021 19:40


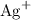 , andSulfide ions
, andSulfide ions 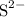 .
.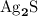 .
.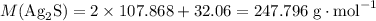 .
.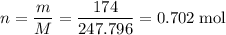 .
. .
.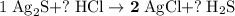 .
.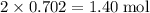 of AgCl.
of AgCl.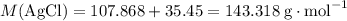 .
.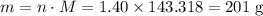 .
.


