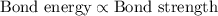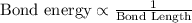Chemistry, 04.02.2020 23:57 cristymarinee9207
Acarbon-carbon double bond has a greater bond energy than a carbon-carbon single bond. because of the greater bond energy, a carbon-carbon double bond is (stronger, weaker) and (longer, shorter) than a carbon-carbon single bond.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Clouds form when water vapor to form small droplets. a. humidifies b. condenses c. evaporates d. precipitates
Answers: 2

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

You know the right answer?
Acarbon-carbon double bond has a greater bond energy than a carbon-carbon single bond. because of th...
Questions



Social Studies, 01.11.2020 14:00




World Languages, 01.11.2020 14:00


Social Studies, 01.11.2020 14:00

Mathematics, 01.11.2020 14:00

English, 01.11.2020 14:00

Business, 01.11.2020 14:00

Mathematics, 01.11.2020 14:00


Social Studies, 01.11.2020 14:00

Social Studies, 01.11.2020 14:00

Computers and Technology, 01.11.2020 14:00

Mathematics, 01.11.2020 14:00


Mathematics, 01.11.2020 14:00