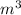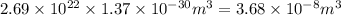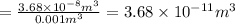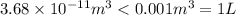Chemistry, 30.01.2020 04:42 FIRENINJA117
Asample of gaseous neon atoms at atmospheric pressure and 0 °c contains 2.69 * 1022 atoms per liter. the atomic radius of neon is 69 pm. what fraction of the space do the atoms occupy? what does this reveal about the separation between atoms in the gaseous phase?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
Asample of gaseous neon atoms at atmospheric pressure and 0 °c contains 2.69 * 1022 atoms per liter....
Questions

Social Studies, 13.01.2021 02:00



Mathematics, 13.01.2021 02:00



Mathematics, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00


English, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00



History, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00



Mathematics, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00

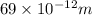
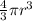
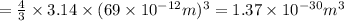
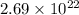 atoms are present in 1L
atoms are present in 1L