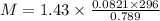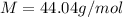Chemistry, 29.01.2020 04:43 kingken3400
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate the molar mass of the gas.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3
You know the right answer?
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate th...
Questions

Mathematics, 27.04.2020 02:23



Mathematics, 27.04.2020 02:23

Social Studies, 27.04.2020 02:23

History, 27.04.2020 02:23

Mathematics, 27.04.2020 02:23


Mathematics, 27.04.2020 02:23

Arts, 27.04.2020 02:23

Mathematics, 27.04.2020 02:23




Mathematics, 27.04.2020 02:23





Geography, 27.04.2020 02:23

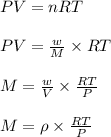
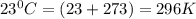
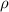 = density =1.43 g/ml
= density =1.43 g/ml