
Chemistry, 28.09.2019 09:20 xmanavongrove55
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for example, a solution of hydrogen peroxide, h2o2, can be titrated against a solution of potassium permanganate, kmno4. the following equation represents the reaction: 2kmno4(aq)+h2o2(aq)+3h2so4(aq)→3o2( g)+2mnso4(aq)+k2so4(aq)+4h2o(l) a certain amount of hydrogen peroxide was dissolved in 100. ml of water and then titrated with 1.68 m kmno4. how much h2o2 was dissolved if the titration required 14.3 ml of the kmno4 solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for...
Questions




Mathematics, 12.07.2019 15:00

Arts, 12.07.2019 15:00




Arts, 12.07.2019 15:00

Mathematics, 12.07.2019 15:00







Mathematics, 12.07.2019 15:00

Biology, 12.07.2019 15:00

Mathematics, 12.07.2019 15:00

Spanish, 12.07.2019 15:00

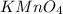 solution = 1.68 M
solution = 1.68 M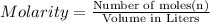
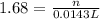
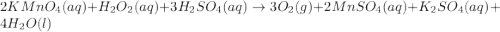
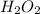 , then 0.0240 moles of KMnO_4 will react with :
, then 0.0240 moles of KMnO_4 will react with :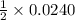 moles of
moles of 

