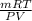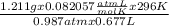Chemistry, 29.01.2020 03:59 svarner2001
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperature in the laboratory is 296 k, and the air pressure is 0.987 atm. calculate the molar mass of the gas.
a) 23.7 g/mol
b) 44.0 g/mol
c) 58.5 g/mol
d) 82.3 g/mol

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
You know the right answer?
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperatu...
Questions





Mathematics, 02.10.2019 14:00


Mathematics, 02.10.2019 14:00


History, 02.10.2019 14:00

Social Studies, 02.10.2019 14:00



Mathematics, 02.10.2019 14:00


Chemistry, 02.10.2019 14:00

Mathematics, 02.10.2019 14:00

Mathematics, 02.10.2019 14:00

Mathematics, 02.10.2019 14:00


History, 02.10.2019 14:00

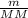 ) RT → MM =
) RT → MM =