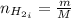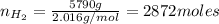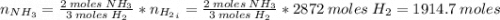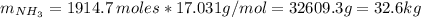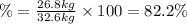Chemistry, 28.09.2019 15:10 victoriacarr638
Areaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 5.79 kg of h2 and excess n2. a total of 26.8 kg of nh3 are produced. what is the percent yield of the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

You know the right answer?
Areaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 5.79 kg...
Questions

Computers and Technology, 25.01.2021 16:10



Chemistry, 25.01.2021 16:10



Mathematics, 25.01.2021 16:10


Mathematics, 25.01.2021 16:10


Mathematics, 25.01.2021 16:10


Mathematics, 25.01.2021 16:10

Physics, 25.01.2021 16:10






English, 25.01.2021 16:10

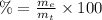 (2)
(2)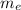 : is the experimental (or actual) mass = 26.8 kg
: is the experimental (or actual) mass = 26.8 kg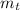 : is the theoretical mass
: is the theoretical mass