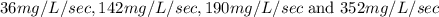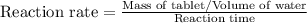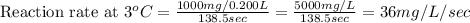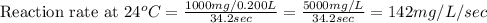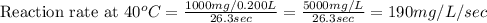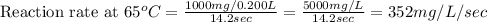Compute reaction rates for all seven trials
reaction rate is usually computed as a change in co...

Chemistry, 27.11.2019 06:31 luvpeaceandsocc3678
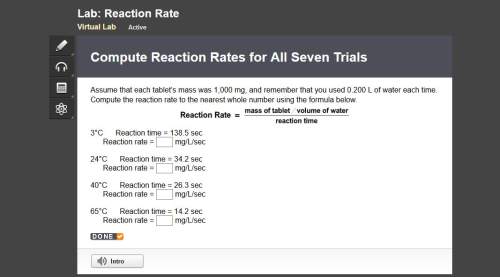
Compute reaction rates for all seven trials
reaction rate is usually computed as a change in concentration (e. g., molarity) per change in time. the tablet is not pure nahco3, so the molarity cannot be computed accurately. (in addition, the quantities in this lab are quite small, and the resulting values would be small decimal values.) instead, compute the following as a measurement of reaction rate.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

You know the right answer?
Questions

English, 03.05.2021 15:30




Mathematics, 03.05.2021 15:30





History, 03.05.2021 15:30

Mathematics, 03.05.2021 15:30


History, 03.05.2021 15:30

Mathematics, 03.05.2021 15:30

Biology, 03.05.2021 15:30





Mathematics, 03.05.2021 15:30

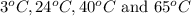 are
are