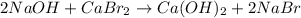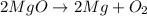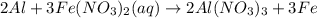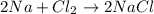Chemistry, 24.09.2019 13:30 sophiav9780
Given the following reactants, determine the type of reaction that will take place, predict the products ,and produce a balanced chemical equation
sodium hydroxide and calcium bromide
magnesium oxide
aluminum and iron (ii) nitrate
sodium and chlorine

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
Given the following reactants, determine the type of reaction that will take place, predict the prod...
Questions


Mathematics, 15.02.2021 15:20

Mathematics, 15.02.2021 15:20



English, 15.02.2021 15:20

Business, 15.02.2021 15:20


Mathematics, 15.02.2021 15:30

English, 15.02.2021 15:30


Geography, 15.02.2021 15:30






Mathematics, 15.02.2021 15:30


Social Studies, 15.02.2021 15:30