
Chemistry, 30.01.2020 07:50 wrerteteT2827
Consider the following reaction at equilibrium: 2co2 (g) ↔ 2co (g) + o2 (g) ∆h° = -514 kj le châtelier's principle predicts that removing o2(g) to the reaction container will consider the following reaction at equilibrium: 2co2 (g) 2co (g) + o2 (g) ∆h° = -514 kj le châtelier's principle predicts that removing o2(g) to the reaction container will decrease the value of the equilibrium constant increase the partial pressure of co increase the value of the equilibrium constant decrease the partial pressure of co increase the partial pressure of co2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

You know the right answer?
Consider the following reaction at equilibrium: 2co2 (g) ↔ 2co (g) + o2 (g) ∆h° = -514 kj le châtel...
Questions

History, 26.08.2019 10:10

Biology, 26.08.2019 10:10

History, 26.08.2019 10:10




Mathematics, 26.08.2019 10:10

English, 26.08.2019 10:10

History, 26.08.2019 10:10





English, 26.08.2019 10:10


Computers and Technology, 26.08.2019 10:10


Chemistry, 26.08.2019 10:10

Social Studies, 26.08.2019 10:10

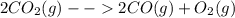 ∆H° = -514
∆H° = -514 

