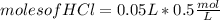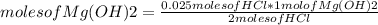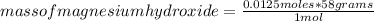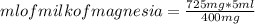Chemistry, 27.09.2019 11:20 bravomichelle75
For every 5.00 ml of milk of magnesia there are 400. mg of magnesium hydroxide. how many ml of milk of magnesia do we need to neutralize? for every 5.00 ml of milk of magnesia there are 400. mg of magnesium hydroxide. how many ml of milk of magnesia do we need to neutralize 50.0 ml of 0.500 m hcl? express your answer in ml.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
For every 5.00 ml of milk of magnesia there are 400. mg of magnesium hydroxide. how many ml of milk...
Questions





English, 08.02.2021 17:30

Biology, 08.02.2021 17:30


Mathematics, 08.02.2021 17:30


Mathematics, 08.02.2021 17:30



Mathematics, 08.02.2021 17:30


Mathematics, 08.02.2021 17:30


English, 08.02.2021 17:30

Mathematics, 08.02.2021 17:30


Chemistry, 08.02.2021 17:30