Chemistry, 05.02.2020 09:53 lanipooh01
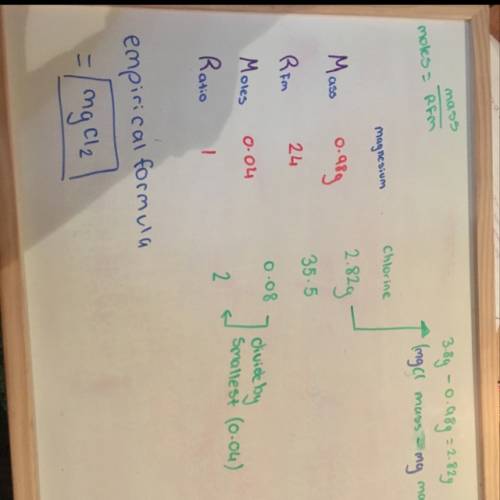
0.98 g of magnesium combined with chlorine gas to form 3.8 g of magnesium chloride. determine the empirical formula of the product.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
0.98 g of magnesium combined with chlorine gas to form 3.8 g of magnesium chloride. determine the em...
Questions

Medicine, 12.12.2020 17:00

Chemistry, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

English, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Geography, 12.12.2020 17:00


Biology, 12.12.2020 17:00

Social Studies, 12.12.2020 17:00

History, 12.12.2020 17:00





Health, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Arts, 12.12.2020 17:00

English, 12.12.2020 17:00