
Chemistry, 05.02.2020 10:46 tintinayeir2567
The equilibrium constant for the reaction 2x(g)+y(g)=2z(g) is 2.25 . what would be the concentration of y at equilibrium with 2 mole of x and 3 mole of z in a one litre vessel?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
The equilibrium constant for the reaction 2x(g)+y(g)=2z(g) is 2.25 . what would be the concentration...
Questions





English, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01



Computers and Technology, 23.09.2020 14:01

History, 23.09.2020 14:01


History, 23.09.2020 14:01



Mathematics, 23.09.2020 14:01




Mathematics, 23.09.2020 14:01

![[\text{Y}] \approx0.337\;\text{mol}\cdot\text{dm}^{-3}](/tpl/images/0503/5677/4561d.png) at equilibrium.
at equilibrium.![[\text{X}] = \dfrac{n}{V} = 2\; \text{mol}\cdot \text{dm}^{-3}](/tpl/images/0503/5677/16f3a.png) ;
;![[\text{Y}] = \dfrac{n}{V} = 0\; \text{mol}\cdot \text{dm}^{-3}](/tpl/images/0503/5677/36c99.png) ;
;![[\text{Z}] = \dfrac{n}{V} = 3\; \text{mol}\cdot \text{dm}^{-3}](/tpl/images/0503/5677/168fc.png) .
.![[\text{Y}]](/tpl/images/0503/5677/40b69.png) be
be 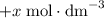 .
. 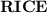 table.
table.![[\text{X}]](/tpl/images/0503/5677/1967d.png) will be
will be 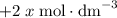 and the change in
and the change in ![[\text{Z}]](/tpl/images/0503/5677/21145.png) will be
will be 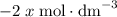 .
.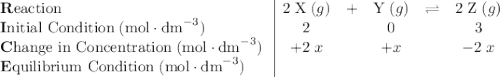 .
.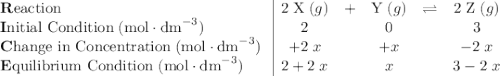 .
.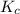 for this reaction? Raise the concentration of each species to its coefficient. Products go to the numerator and reactants are on the denominator.
for this reaction? Raise the concentration of each species to its coefficient. Products go to the numerator and reactants are on the denominator. ![K_c = \dfrac{[\text{Z}]^{2}}{[\text{X}]^{2} \cdot[\text{Y}]}](/tpl/images/0503/5677/260f1.png) .
.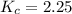 . As a result,
. As a result,![\dfrac{[\text{Z}]^{2}}{[\text{X}]^{2} \cdot[\text{Y}]} = \dfrac{(3-2x)^{2}}{(2+2x)^{2} \cdot x} = K_c = 2.25](/tpl/images/0503/5677/bc2d1.png) .
.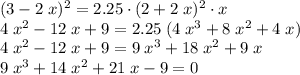 .
.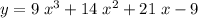 on a graph and look for any zeros. There's only one zero at
on a graph and look for any zeros. There's only one zero at 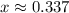 . All three concentrations end up greater than zero.
. All three concentrations end up greater than zero. 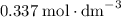 .
.

