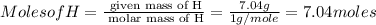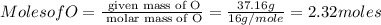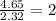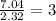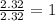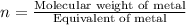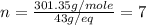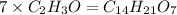Chemistry, 01.10.2019 17:30 manaisialockhart12
Asubstance has 55.80% carbon, 7.04% hydrogen, and 37.16% oxygen. what is it's empirical and molecular formula if it has a molar mass of 301.35 grams

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

You know the right answer?
Asubstance has 55.80% carbon, 7.04% hydrogen, and 37.16% oxygen. what is it's empirical and molecula...
Questions



SAT, 11.01.2021 03:30


Mathematics, 11.01.2021 03:30





Biology, 11.01.2021 03:30

Mathematics, 11.01.2021 03:30

Mathematics, 11.01.2021 03:30



Mathematics, 11.01.2021 03:30

Mathematics, 11.01.2021 03:30

History, 11.01.2021 03:30


Mathematics, 11.01.2021 03:30

Biology, 11.01.2021 03:30

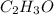 and molecular formula is
and molecular formula is 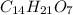
![Moles of C =[tex] \frac{\text{ given mass of C}}{\text{ molar mass of C}}= \frac{55.80g}{12g/mole}=4.65moles](/tpl/images/0280/2916/25d2c.png)