
Chemistry, 21.11.2019 09:31 rubianny03
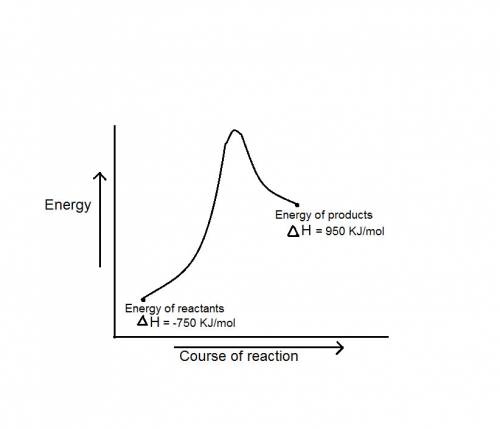
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statement is correct about the reaction?
it is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2
You know the right answer?
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statemen...
Questions








Chemistry, 13.10.2020 04:01





Mathematics, 13.10.2020 04:01


History, 13.10.2020 04:01

Mathematics, 13.10.2020 04:01


English, 13.10.2020 04:01


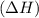 comes out to be positive.
comes out to be positive.

