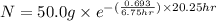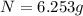Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
The half life of pa-234 is 6.75 hr. how much of a 50.0g sample of this isotope remains after 20.25 h...
Questions


Mathematics, 04.02.2021 05:40



Spanish, 04.02.2021 05:40


Mathematics, 04.02.2021 05:40



Mathematics, 04.02.2021 05:40

Mathematics, 04.02.2021 05:40




Mathematics, 04.02.2021 05:40



Mathematics, 04.02.2021 05:40


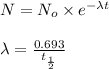
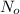 = initial mass of isotope
= initial mass of isotope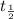 = half life of the isotope = 6.75 hr
= half life of the isotope = 6.75 hr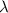 = rate constant
= rate constant