
Chemistry, 28.01.2020 07:31 squiddddplop
Plz answer concentration of either the h+ ion or the oh − ion is given for four aqueous solutions at 298 k.?
for each solution, calculate [h+] or [oh − ]. state whether the solution is acidic, basic, or neutral.
(a) [h+] = 8.0 * 10^-13 m
[oh − ] =
(b) [oh − ] = 4.0 * 10^-7 m
[h+] =
(c) [oh − ] = 5.0 * 10^-3 m
[h+] =
(d) [h+] = 8.0 * 10^-5 m
[oh − ] =

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Plz answer concentration of either the h+ ion or the oh − ion is given for four aqueous solutions a...
Questions

Mathematics, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00



Mathematics, 13.11.2020 01:00

English, 13.11.2020 01:00

English, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00


Mathematics, 13.11.2020 01:00

Spanish, 13.11.2020 01:00



Mathematics, 13.11.2020 01:00

Business, 13.11.2020 01:00

Biology, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00

History, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00

German, 13.11.2020 01:00

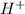 =
= 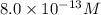
![[H^+][OH^-]=10^{-14}](/tpl/images/0475/8440/7d81f.png)
![(8.0\times 10^{-13})\times [OH^-]=10^{-14}](/tpl/images/0475/8440/5e33b.png)
![[OH^-]=1.25\times 10^{-2}M](/tpl/images/0475/8440/54850.png)
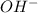 =
= 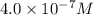
![[H^+]\times (4.0\times 10^{-7})=10^{-14}](/tpl/images/0475/8440/9d559.png)
![[H^+]=0.25\times 10^{-7}M](/tpl/images/0475/8440/b9c16.png)
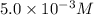
![[H^+]\times (3.0\times 10^{-3})=10^{-14}](/tpl/images/0475/8440/f3c6a.png)
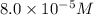
![(8.0\times 10^{-5})\times [OH^-]=10^{-14}](/tpl/images/0475/8440/87df8.png)


