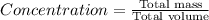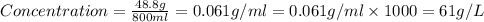Read the statement.
a 200 ml nacl solution with a concentration of 4.0 g/l is mixed wit...

Chemistry, 28.09.2019 01:00 trevorhenyan51
Read the statement.
a 200 ml nacl solution with a concentration of 4.0 g/l is mixed with a 600 ml solution containing 8% nacl (m/v).
what is the final concentration of salt in the solution in g/l?
a.4.7
b.61
c.6.1
d.47

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1


Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Questions

English, 26.04.2020 00:43

Mathematics, 26.04.2020 00:44

Mathematics, 26.04.2020 00:44


Mathematics, 26.04.2020 00:45

Mathematics, 26.04.2020 00:45

Mathematics, 26.04.2020 00:45









Biology, 26.04.2020 00:47



Social Studies, 26.04.2020 00:47

Mathematics, 26.04.2020 00:50

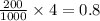 grams of mass of NaCl
grams of mass of NaCl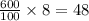 grams of NaCl
grams of NaCl