
Chemistry, 06.10.2019 07:11 kberly3750ovgw6f
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be formed by reacting 4 atoms of iron (fe) with 3 molecules of oxygen gas (o2). if 12 atoms of iron are reacted with 6 molecules of oxygen gas, which is the limiting reactant and how many atoms or molecules will be left over? 4fe + 3o2 -> 2fe2o3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2


Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be for...
Questions

Social Studies, 07.07.2019 13:20

History, 07.07.2019 13:20



History, 07.07.2019 13:20

Social Studies, 07.07.2019 13:20

Business, 07.07.2019 13:20

Chemistry, 07.07.2019 13:20

Business, 07.07.2019 13:20

History, 07.07.2019 13:20

History, 07.07.2019 13:20

Social Studies, 07.07.2019 13:20

Mathematics, 07.07.2019 13:20

Mathematics, 07.07.2019 13:20

Mathematics, 07.07.2019 13:20



History, 07.07.2019 13:20


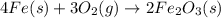
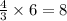 atoms of iron.
atoms of iron.

