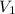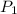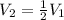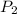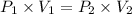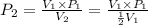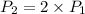Chemistry, 12.01.2020 01:31 middlegirlrule6848
The volume of a gas decreases to half of its original volume, but the gas maintains the same number of moles and temperature. according to the ideal gas law, what will most likely happen to the pressure?
a.)it will double.
b.)it will decrease.
c.)it will increase slightly.
d.)it will remain the same.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
The volume of a gas decreases to half of its original volume, but the gas maintains the same number...
Questions

Biology, 01.03.2021 21:20

Mathematics, 01.03.2021 21:20

English, 01.03.2021 21:20


English, 01.03.2021 21:20










English, 01.03.2021 21:30

Health, 01.03.2021 21:30


Computers and Technology, 01.03.2021 21:30


Business, 01.03.2021 21:30