
The following reaction occurs in a car’s catalytic converter.
2no(g) + 2co(g) ==> n2(g) + 2co2(g)
which answer best describes the reducing and oxidizing processes in this reaction?
no and co are both reducing agents.
no and co are both oxidizing agents.
the oxidation state of nitrogen in no changes from +2 to 0, and the oxidation state of carbon in co changes from +2 to +4 as the reaction proceeds.
the oxidation state of nitrogen in no changes from 0 to +2, and the oxidation state of carbon in co changes from +4 to +2 as the reaction proceeds.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
The following reaction occurs in a car’s catalytic converter.
2no(g) + 2co(g) ==> n2...
2no(g) + 2co(g) ==> n2...
Questions


Mathematics, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00

Chemistry, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00


English, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00

Health, 26.03.2021 08:00

Geography, 26.03.2021 08:00

History, 26.03.2021 08:00

English, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00

Mathematics, 26.03.2021 08:00



History, 26.03.2021 08:00

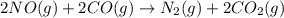
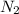 molecule. Hence, nitrogen is getting reduced that is reduction reaction. NO is oxidizing agent
molecule. Hence, nitrogen is getting reduced that is reduction reaction. NO is oxidizing agent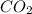 molecule. Hence ,carbon is getting oxidized that is oxidation reaction. CO is a reducing agent.
molecule. Hence ,carbon is getting oxidized that is oxidation reaction. CO is a reducing agent.

