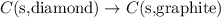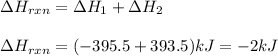Chemistry, 11.01.2020 03:31 mallorybranham
Given the equations below, which description applies to the conversion of diamond to graphite? c(s, diamond) + o2 (g) → co2 (g), ∆h = –395.4 kj co2 (g) → c(s, graphite) + o2 (g), ∆h = 393.5 kj explain your answer
a) energy is created during the process.
b) heat is neither released nor absorbed during the process.
c) heat is released during the process.
d) the process is endothermic.

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

You know the right answer?
Given the equations below, which description applies to the conversion of diamond to graphite? c(s,...
Questions


Computers and Technology, 29.08.2019 16:30

Social Studies, 29.08.2019 16:30





Mathematics, 29.08.2019 16:30



Biology, 29.08.2019 16:30





Mathematics, 29.08.2019 16:30

Chemistry, 29.08.2019 16:30



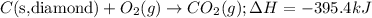 ....(1)
....(1)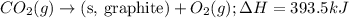 ....(2)
....(2)