
Chemistry, 20.01.2020 10:31 ayoismeisalex
What must be the molarity of an aqueous solution of trimethylamine, (ch3)3n, if it has a ph = 11.20? (ch3)3n+h2o⇌(ch3)3nh++oh−kb=6.3×10− 5?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 08:30
This has nothing to do with school. i wrote a poem to my crush, who i'm asking out soon. tell me if it's cheesy, or cute. "roses are red, violets are blue no love story sounds right if it doesn't include you. dance with me all night, gaze into my eyes i'll hand you my heart, as well as my pride. when i hear your name, my heart goes insane. your all that i want, all that i need promise me you'll stay with me. here it is the final line, jasmine hill will you be mine? " i'm also going to buy her flowers, teddy bear and some food lol. written by me, bre (:
Answers: 2


Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2
You know the right answer?
What must be the molarity of an aqueous solution of trimethylamine, (ch3)3n, if it has a ph = 11.20?...
Questions

English, 28.09.2019 15:30


History, 28.09.2019 15:30


Mathematics, 28.09.2019 15:30


Social Studies, 28.09.2019 15:30


Mathematics, 28.09.2019 15:30

History, 28.09.2019 15:30

Chemistry, 28.09.2019 15:30

English, 28.09.2019 15:30

Biology, 28.09.2019 15:30


History, 28.09.2019 15:30

Mathematics, 28.09.2019 15:30


Biology, 28.09.2019 15:30


Biology, 28.09.2019 15:30

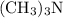 in this question acts as a weak base. As seen in the equation in the question,
in this question acts as a weak base. As seen in the equation in the question, 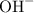 rather than
rather than 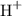 when it dissolves in water. The concentration of
when it dissolves in water. The concentration of ![[\text{OH}^{-}]](/tpl/images/0462/5453/3da65.png) from pH:
from pH: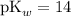 ,
, .
.![[\text{OH}^{-}] =10^{-\text{pOH}} =10^{-2.80} = 1.59\;\text{mol}\cdot\text{dm}^{-3}](/tpl/images/0462/5453/5eaa9.png) .
.![[(\text{CH}_3)_3\text{N}]_\text{initial}](/tpl/images/0462/5453/27976.png) :
:![\dfrac{[\text{OH}^{-}]_\text{equilibrium}\cdot[(\text{CH}_3)_3\text{NH}^{+}]_\text{equilibrium}}{[(\text{CH}_3)_3\text{N}]_\text{equilibrium}} = \text{K}_b = 1.58\times 10^{-3}](/tpl/images/0462/5453/44236.png)
![[(\text{CH}_3)_3\text{N}]_\text{initial} = [(\text{CH}_3)_3\text{N}]_\text{final}](/tpl/images/0462/5453/e63b0.png) .
.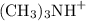 was also produced. The solution started with a small amount of either species. As a result,
was also produced. The solution started with a small amount of either species. As a result, ![[(\text{CH}_3)_3\text{NH}^{+}] = [\text{OH}^{-}] = 10^{-2.80} = 1.58\times 10^{-3}\;\text{mol}\cdot\text{dm}^{-3}](/tpl/images/0462/5453/587e5.png) .
.![\dfrac{[\text{OH}^{-}]_\text{equilibrium}\cdot[(\text{CH}_3)_3\text{NH}^{+}]_\text{equilibrium}}{[(\text{CH}_3)_3\text{N}]_\textbf{initial}} = \text{K}_b = 1.58\times 10^{-3}](/tpl/images/0462/5453/be689.png) ,
,![[(\text{CH}_3)_3\text{N}]_\textbf{initial} =\dfrac{[\text{OH}^{-}]_\text{equilibrium}\cdot[(\text{CH}_3)_3\text{NH}^{+}]_\text{equilibrium}}{\text{K}_b}](/tpl/images/0462/5453/90893.png) ,
,![[(\text{CH}_3)_3\text{N}]_\text{initial} =\dfrac{(1.58\times10^{-3})^{2}}{6.3\times10^{-5}} = 0.040\;\text{mol}\cdot\text{dm}^{-3}](/tpl/images/0462/5453/76ffc.png) .
.

