An aqueous solution is 15.0% by mass of copper(ii) sulfate pentahydrate, cuso4∙5h2o. what
is th...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
Questions

Chemistry, 13.11.2019 16:31

History, 13.11.2019 16:31

Geography, 13.11.2019 16:31



Mathematics, 13.11.2019 16:31

Social Studies, 13.11.2019 16:31


Mathematics, 13.11.2019 16:31



Mathematics, 13.11.2019 16:31

Health, 13.11.2019 16:31




Mathematics, 13.11.2019 16:31


Social Studies, 13.11.2019 16:31

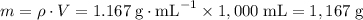 .
.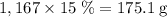 among that 1,167 grams of the solution is
among that 1,167 grams of the solution is 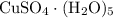 .
. 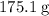 of the hydrate:
of the hydrate: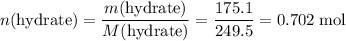 .
.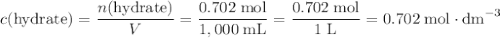 .
.



