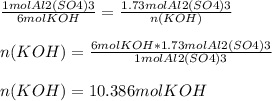Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 12:20
Describe the structure of ammonium lauryl sulfate. refer to the given diagram. your answer should include the type of bonding, the elements contained, and the size and shape of the molecule. write a short paragraph.
Answers: 3
You know the right answer?
How many moles of potassium hydroxide are needed to completely react with 1.73 miles of aluminum sul...
Questions

Mathematics, 11.11.2019 15:31

Mathematics, 11.11.2019 15:31

English, 11.11.2019 15:31


English, 11.11.2019 15:31

Mathematics, 11.11.2019 15:31



Biology, 11.11.2019 15:31



History, 11.11.2019 15:31


Social Studies, 11.11.2019 15:31

Mathematics, 11.11.2019 15:31