
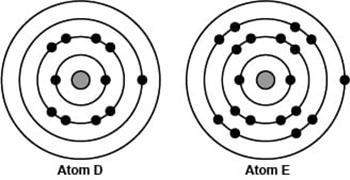
The image compares the arrangement of electrons in two different neutral atoms.
which of the following best explains the position of the two atoms in the periodic table?
a)both atoms have an estimated zeff of 1; therefore, atom d is to the right of atom e in the same period.
b)both atoms have zeff of 1; therefore, atom d is above atom e in the same column because of the additional energy level.
c)atom d has an estimated zeff of 1 and is therefore to the left of atom e, which has a zeff of 9.
d)atom d has an estimated zeff of 1 and is therefore below atom e in the same column, which has a zeff of 9.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
The image compares the arrangement of electrons in two different neutral atoms.
which of...
which of...
Questions

Mathematics, 16.12.2020 06:10

History, 16.12.2020 06:10

Chemistry, 16.12.2020 06:10



Mathematics, 16.12.2020 06:10


English, 16.12.2020 06:10

Mathematics, 16.12.2020 06:10




Mathematics, 16.12.2020 06:20

Law, 16.12.2020 06:20



Advanced Placement (AP), 16.12.2020 06:20



Chemistry, 16.12.2020 06:20



