
Chemistry, 24.11.2019 11:31 jdvazquez18p7a7vs
Find the concentration of h+ ions at a ph = 11 and ph = 6. then divide the concentration of h+ ions at a ph = 11 by the of h+ ions at a ph = 6. record your answer in table c. what is the concentration of h+ ions at a ph = 11? mol/l what is the concentration of h+ ions at a ph = 6? mol/l how many fewer h+ ions are there in a solution at a ph = 11 than in a solution at a ph = 6?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Find the concentration of h+ ions at a ph = 11 and ph = 6. then divide the concentration of h+ ions...
Questions


History, 30.10.2020 19:50


Mathematics, 30.10.2020 19:50


English, 30.10.2020 19:50

Biology, 30.10.2020 19:50



English, 30.10.2020 19:50


Chemistry, 30.10.2020 19:50

English, 30.10.2020 19:50

Biology, 30.10.2020 19:50


Mathematics, 30.10.2020 19:50


Mathematics, 30.10.2020 19:50


![pH=-\log[H^+]](/tpl/images/0388/8114/cf945.png)
![11=-\log[H^+]](/tpl/images/0388/8114/c91a3.png)
![[H^+]=1\times 10^{-11} M](/tpl/images/0388/8114/212e9.png) ..(1)
..(1)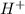 ions is
ions is 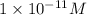 .
.![pH=-\log[H^+]'](/tpl/images/0388/8114/8468d.png)
![6=-\log[H^+]'](/tpl/images/0388/8114/502fa.png)
![[H^+]'=1\times 10^{-6} M](/tpl/images/0388/8114/628fb.png) ..(2)
..(2)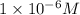 .
.![\frac{[H^+]}{[H^+]'}=\frac{1\times 10^{-11} M}{1\times 10^{-6}}=1\times 10^{-5}](/tpl/images/0388/8114/bd462.png)
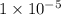 .
.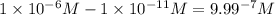
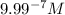 ions fewer than in a solution at a pH = 6
ions fewer than in a solution at a pH = 6

